ホーム > ENGLISH
What is Japan Food Additives Association?
(Ⅰ)OUTLINE
Japan Food Additives Association (JAFA) was established in September 1982 as a nation-wide consolidated body, founding on both Tokyo Food Additives Association and Nippon Food Additives Group Leagues. JAFA is composed of companies and associations that are involved in the production, import, sales and utilization of food additives. In April 2014, JAFA was registered as a general incorporated association, and since then, JAFA takes new responsibilities.
The objective of JAFA is to serve both to its members and the public by sharing information about the safety and benefit of food additives under the guidance of Food Safety Standards and Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau, Ministry of Health, Labor and Welfare and consequently, to contribute to the sound development of food and food additives industry and to the safe and healthy life of consumers.

(Ⅱ)ORGANIZATION
JAFA currently has about 950 members and is managed by a chairman, two vice chairmen, an executive director and five managing directors. The general secretariat of JAFA is located in Tokyo (Head Office) and Osaka (Branch).
The activities are carried out with the coordination of five General Subject Committees in charge of administrative affairs, technical and regulatory affairs, safety and international affairs, public relations affairs and quality assurance affairs, respectively. As Category Committees, there are 14 groups that conduct investigations, research and studies for designated category of food additives:
Group 1 Sweeteners
Group 2 Colors
Group 3 Preservatives, Antimould Agents, etc
Group 4 Stabilizers, Thickeners
Group 5 Antioxidant, Vitamins
Group 6 Gum-base, Glazing agents
Group 7 Enzymes
Group 8 Acids, pH adjusting agents
Group 9 Seasonings, amino acids, etc
Group 10 Emulsifiers
Group 11 Yeast foods, Raising agents, etc
Group 12 Phosphates
Group 13 Processing agents
Group 14 Flavors, Solvents, Spices
The local chapters (in Sendai, Nagoya, Hiroshima and Fukuoka area) coordinate their activities with the local governmental bodies to act as a liaison among the regional member companies.

(Ⅲ)ACTIVITIES
- Monthly seminars conveying information to the members by the heads of the five general subject committees are held both at Tokyo and Osaka to let the members keep in close contact and exchange information each other. Technical seminars and training programs for employees of the members by expert lecturers are undertaken. General assemblies are held at the chapters and occasional seminars are carried out as needed.
- Through close communication and guidance from Ministry of Health, Labor and Welfare and other relevant authorities, the governmental and regulatory information on food additives is quickly disseminated to the members, and requests from member companies are conveyed to the relevant authorities.
- Technical information regarding safety and benefits of food additives is collected and analyzed on an international level.
- JAFA responds to inquiries and requests from member companies for studies regarding the safety and usefulness of food additives.
- Information concerning the safety and benefits of food additives is maintained for consumers, and communication and educational activities directed toward consumer groups are carried out. Furthermore, informative pamphlets, brochures and videos on food additives are prepared and distributed to provide accurate knowledge on these subjects.
- A scientific journal, Japan Food Additives News (JAFAN) is published bi-monthly and an activity report and news letters, Japan Food Additives Association Report, is published monthly for the members.
- With close cooperation with Ministry of Health, Labor and Welfare and National Institute of Health Science, new specifications and standards for existing food additives are developed to incorporate to "The Japanese Specifications and Standards for Food Additives". And newly developed specifications and standards are translated into English with cooperation with National Institute of Health Science.
- JAFA runs voluntary certification program for KANSUI (an alkaline preparation for Chinese noodles) and tar color preparations. In addition, JAFA also promotes self-responsible management of the food additives industry by preparing voluntary specifications of food additives and a responsible authorization system of food additives good manufacturing practice (GMP).
Food Additives
by Japan Food Additives Association (ver1.2020 Mar 16)
The aim of this document is to outlines Japan’s regulations on food additives.
Part I explains about the history of relevant regulations related either of the Ministry of Health, Labour, and Welfare (MHLW), the Food Safety Commission (FSC), and the Consumer Affairs Agent (CAA).
Part II deals with the definition and categorization of food additives, and Japan’s Specifications and Standards, as well.
Part III outlines the procedure for designation of a new food additive and revision of existing specifications.
Part IV summarizes the labeling of food additives used for manufacturing processed foods.
In the end, but not least, the information in this document is prepared for just your assistance in understanding Japan’s regulation on food additives. In case you would prepare the dossier to submit to Japan’s government, please consult appropriate bodies for confirmation.
Part I. History of Food Additive Regulations
In 1947, Ministry of Health and Welfare (currently Ministry of Health, Labour and Welfare (MHLW)) enacted the Food Sanitation Act (食品衛生法:FSA) as the first comprehensive Act for food safety/hygiene, and introduced a positive list system for food additives. Under the system, only additives designated as safe by the Minister of Health, Labour and Welfare may be used in foods. Since 1947, all food additives have been regulated by this act. However, designation system had been applied only to chemically synthesized additives until 1995 when the FSA was amended. At present, all types of additives are equally subject to the designation system, regardless of synthetic or natural origin, with some exceptions.
After the outbreak of Bovine Spongiform Encephalopathy (BSE) in 2001, the Food Safety Basic Act (食品安全基本法:FSBA) was enacted in 2003 to secure safe foods ad protect the health of consumers. Based on the Basic Law on Food Safety, the Food Safety Commission (FSC) was set up and the risk analysis system was introduced. In this system, the MHLW takes responsibility in risk management, the FSC responsibility in risk analysis, and each responsibility in risk communication with related parties.
In 2009, the Consumer Affairs Agency (CAA) was established as an organization to make and implement the policies aiming to create a safe, worry-free and livable society for consumers. The CAA task includes management of product labelling and thus, the CAA enacted Food Labelling Act in 2015, taking the responsibility on food additive labelling out from the FSA of the MHLW. Consequently, the new CAA regulation was issued.
Part II. Definition and Categories of Food Additives and
II-1. Definition
The Food Sanitation Act (FSA) in the first Chapter defines ''food additive'' as (i) substances used in or on food in the process of manufacturing food, and (ii) substances used for the purpose of processing or preserving food. Consequently, ''food additive'' includes substances remaining in the final products, such as food colors and preservatives, and substances not remaining in the final products, such as microorganism control agents and filtration aids. Regardless of whether they are from natural origin, all substances used for the above purposes are categorized as food additives in Japan.
The scope of food additives referred to by the FSA is different from that defined by the Codex Alimentarius Commission (CAC). The substances below, which are not defined by the CAC as food additives, are all categorized as food additives in Japan.
(i) Processing aids,* like infiltration-supporting agents
(ii) Vitamins, minerals, and amino acids
* Processing aid means any substance or material, not including apparatus or utensils, and not consumed as a food ingredient by itself, intentionally used in the processing of raw materials, foods or its ingredients, to fulfill a certain technological purpose during treatment or processing and which may result in the non-intentional but unavoidable presence of residues or derivatives in the final product (CAC, Procedural Manual, “Section I : Definitions for the purpose of the Codex Alimentarius”)
II-2. Categories
In Japan, there are four categories for food additives (Figure I). Among them, the numbers of the designated additives are restricted by the designation system (see below). And the numbers of existing additives are too restricted by its historical reason (see below).
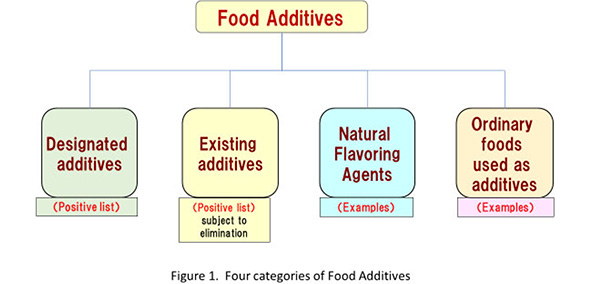
- Designated additive
Designated food additive are substances designated by the Minister of Health, Labor and Welfare based on Article 10 of the Food Sanitation Act (FSA), after risk assessment of Food Safety Commission. This designation includes natural products as well as chemically synthesized products. Further, flavors falling into Class 18 category specified by collective names such as "esters" are indicated in the notice
(List of Class 18 category flavors:
https://www.ffcr.or.jp/tenka/list/post-140.html).
(List of designated additives beside flavors)
https://www.ffcr.or.jp/tenka/list/post-11.html - Existing additives
When the FSA was amended in 1995 to expand the target of the designation from chemically synthesized products to all additives including naturally derived products, additives with a long history of eating and use in Japan have been approved for use and sale as exception. These additives are referred to as existing additives. Confirmation of the safety of existing additives and establishment of standards have been promoted.
(List of existing additives)
https://www.ffcr.or.jp/tenka/list/post-12.html - Standards for Use of Food Additives
Food additives with use standards (i.e., target foods and maximum use limits/residue limits) shall meet these standards, when these substances are used.
https://www.ffcr.or.jp/webupload/e3984852a08b38bffaa3d166a8176173c3916dd2.pdf
The rest of the four categories are natural flavors and ordinary foods used as additives, there are a lot of of natural flavor sources and food sources. So that, the lists show some examples. - Natural flavors
These substances are natural products obtained from animals and plants and used to add flavor to foods. The amounts used are generally in very small.
(Natural fragrance base substance list)
https://www.ffcr.or.jp/shokuhin/upload/%E5%A4%A9%E7%84%B6%E9%A6%99%E6%96%99%E3%83%AA%E3%82%B9%E3%83%88.pdf - Ordinary foods used as additives
They are generally consumed as foods and drinks and also used as food additives (e.g. strawberry juice for coloring, or agar for stabilizing).
(List of ordinary foods used as additives)
https://www.ffcr.or.jp/shokuhin/upload/%E4%B8%80%E8%88%AC%E6%B7%BB%E5%8A%A0%E7%89%A9%E3%83%AA%E3%82%B9%E3%83%88.pdf
II-3. Japan’s Specifications and Standards for Food Additives
The FSA mandates the Minister of Health, Labour and Welfare to prepare an official compilation of food additive specifications and standards. The compilation contains compositional specifications for individual additives as well as standards for manufacturing and use of these additives. The compilation is updated every several years to introduce new and improved test methods commensurate with the progress in science and technology, and to achieve international harmonization of standards. Japan’s Specifications and Standards for Food Additives is the English translation of the official compilation of food additives.
http://www.nihs.go.jp/dfa/dfa-j/shokuten_kikaku_j.html
Part III. Designation of food additives or revision of standards
The designation of a new food additive and the revision of standards for food additives both require risk assessment by the Food Safety Commission (FSC) of Japan and review by the MHLW based on the risk assessment by the FSC.
Those wishing to apply for designation of a food additive or revision of standards for food additives should collect documents required for the application and submit them together with the application form to the MHLW. For details for the application procedure, please consult "The Procedure for Preparing Application Documents for Designation of Food Additives and Revision of Use Standards for Food Additives" (食品添加物の指定及び使用基準改正の要請資料作成に関する手引) and four guidelines for FSC’s risk assessment. Period necessary for approval is outlined in the notification (The standard period required to designate food additives (PDF:63KB))
The procedure is available at (PDF:696KB)
Guidelines for Risk Assessment
Guidelines for the Risk Assessment of Food Additives (Amended Guidelines for Assessment of the Effect of Food on Human Health Regarding Food Additives)
https://www.fsc.go.jp/senmon/tenkabutu/index.data/For_HP_Guidelines_for_Food_Additives.pdf
Guideline for the Assessment of Flavoring Substances in Foods on Health
https://www.fsc.go.jp/senmon/tenkabutu/index.data/GAFSFH.pdf
Guidelines for the Risk Assessment of Additives (Enzymes) in Foods
https://www.fsc.go.jp/senmon/tenkabutu/index.data/For_HP_Guidelines_for_Enzymes.pdf
Guidelines for the Risk Assessment of Food Additives for Fortification
https://www.fsc.go.jp/senmon/tenkabutu/index.data/For_HP_Guidelines_for_FAF.pdf
Guidelines for FSC’s risk assessment are available at
http://www.fsc.go.jp/english/standardsforriskassessment/guideline_assessment_foodadditives_e2.pdf
For verbal consultation of application for designation of food additives or revision of standards, please contact Food Additive Designation Consultation Center (FADCC) of National Institute of Health Sciences.
https://www.nihs.go.jp/dfa/dfa-fadcc/e_fadcc/fadcc_ehome.html
Data of average intake of individual food―additional data to the survey report on food intake and frequency (食品摂取頻度・摂取量調査の特別集計業務 報告書追加資料)
―is available at (PDF:899KB) (xls:1,004KB)
Notice: If the applicant lives overseas, he or she should establish a contact person in Japan for responsible handling of the application.


Part IV. Labeling of Food Additives on Foods
With regard to the labeling of additives in foods, the jurisdiction was transferred to the Consumer Affairs Agency (CAA) in 2009 and a notification (CAA Food Labeling Div. Notification No. 377, October 20, 2010) was issued shortly after. In 2015 the CAA enacted the Food Label Act (食品表示法), and the Food Label Regulation (食品表示基準) by consolidating the labeling regulations from three separate acts; namely, the FSA, the Japan Agricultural Standard Act (JAS法) and Health Promotion Act (健康増進法).
Regarding the food additives listed in the attachment of the notification (CAA Food Labeling Div. Notification No. 8, January 15, 2020), the notification states that “such additives used in foods” shall be labeled (in principle, by “substance name”) on the food (Food Labeling Regulation, Chapter 2, Section 1, Subsection 1, “Processed Foods for Consumer”, Article 3). Besides the substance names, there are two different ways to label food additives (for details, see below). Additives used as nutrition fortification agents, processing aids and carryovers are excluded from the label.
The above requirements apply to food additives contained in foods subject to ministerial ordinances on milk and dairy products regarding composition standards.
“Nutrition fortification agents” are substances added for nutritional purposes during the processing of foods. They are exempt from labeling (excluding infant formulas). However, when used for purpose other than fortification, labeling of the substance name is required for fortifiers (Food Labeling Regulation, Chapter 2, Section 1, Subsection 1 “General Use Processed Foods”)
“Processing aids” are substances added during the processing of food, which are removed before the food is completed, and are the same as the ingredients normally contained in the food due to the raw materials of the food. It refers to a substance that is converted into an ingredient and does not significantly increase the amount of the ingredient, and that is contained in the food in a small amount and does not affect the food by the ingredient.
"Carryover" is a substance used in the manufacture or processing of food ingredients and is not used in the process of manufacturing or processing the food, and the additive is effective in the food. Is less than the amount that can be contained.
IV-1. Labeling methods
In an ingredient list, food additives are requested to be labeled separately from the rests of food ingredients (in principle,) by making a new line (Food Labeling Regulation, Chapter 2, Section 1, Subsection 1, “Processed Foods for Consumer”, Article 8). There are alternative ways to label food additives by indicating in a new box or a slash “/”. Labeling of food additives used in foods is done in the following order, in descending order of weight in the additive. Labeling was done by either of the following manner, substance name and category combination or collective name.
- Designated additives
Use the name described in the Annex “Additive 1-1” to the notification (CAA Food Labeling Div. Notification No. 8, January 15, 2020). Abbreviated name and categorized name appeared in the Annex can be also used in the labeling. When several additives having the same functions are used in combination, they can be displayed in a simple manner, as shown in “Additive 1-2” of the notice.
Example: When lactic acid, sodium lactate and calcium lactate are used in combination, “lactic acid (Na, Ca)” - Existing additives
Additives in the list of items on the list of existing additives (Annex "Additives 2-1" in the notification (CAA Food Labeling Div. Notification No. 8, January 15, 2020)) It shall be displayed with the name described in. Labeling can also be performed for each property name, alias, abbreviation, or class name described in “Additive 2-1” above. - Natural flavoring agents
The labeling of the substance shall be based on the basic substance described in Annex “Additives 2-2” in the aforementioned notification. Performed by first name or alias. Labels must be accompanied by fragrances. "Additive 2-2" is an example, and labeling of natural fragrances not listed in this table is performed with scientifically appropriate names that can identify these additives. - Substances generally provided as food and used as additives
These are called general food additives, and are described in Annex “Additives 2-3” in the above-mentioned notification. Have been. These indications are made using the names (substance names) or the short names described in “Additives 2-3”. "Additives 2-3" are exemplary and substances not listed in this table are labeled with scientifically relevant names by which these additives can be identified. - Labeling by substance names and category name in combination
Additives used as fungicides, antioxidants, bleaching agents, color fixing agents, flavoring agents, preservatives, sweeteners, thickeners/stabilizers/gelling agents/thickeners are indicated by the substance name. (Food Labeling Regulation, Chapter 2, Section 1, Subsection 1, “Processed Foods for Consumer,” Article 3, Schedule 6). However, in the case of an additive used for coloring purposes, if the character of “color” (color) by the substance name is included, the use name can be omitted. Similarly, in the case of a thickener or a paste, when the substance name includes the characters "thickening" (thickening), the use name can be omitted. - Labeling by collective name
Among the additives, the following can be alternatively indicated by their names: yeast food, gum base, Kansui (an alkaline preparation for Chinese noodles), enzymes, brighteners, flavors, acidulants, softeners (Chewing gums only), seasonings, coagulants for tofu (tofu), bitters, emulsifiers, pH adjusters and swelling agents (Food Labeling Regulation, Chapter 2, Section 1, Subsection 1, “Processed Foods for Consumer,” Article 3, Schedule 7).
Regarding seasonings: If the substance is composed only of amino acids, the label will be "seasonings (amino acids)". If the substance is mainly composed of amino acids, the label is "seasoning (such as amino acids)". If the substance consists only of organic acids, the label will be "seasoning (organic acids)". When the substance is mainly composed of an inorganic acid, the label is "seasoning (inorganic acid or the like)". The swelling agent can be designated as a swelling agent, baking powder or leavening powder. Perfumes can be displayed as perfumes or synthetic perfumes.
IV-2. Notice for labeling
(1) It is forbidden to claim “natural” or any expression implying “natural” in the labeling of additives.
(2) Imazalil, 0-phenylphenol, sodium 0–phenylphenate, diphenyl or thiabendazole used in citrus fruits and bananas, which are sold loose, must be labeled.
(3) Labeling by substance name, abbreviation or by class name shall be done in principle by name indicated in the notification (CAA Food Labeling Div. Notification No. 8, January 15, 2020). However, it may be done using hiragana, katakana or Chinese characters, so long as the indication will not be misunderstood by consumers.
IV-3. Omission of labeling
Labeling can be omitted for those products in a container/package with a surface area of not more than 30 cm2. However, the following items shall be appeared on the food label: product name, preservation method, expiration date or perishable date, food business operator’s name and address and allergen and L-phenylalanine compound, as appropriate.
endLink
- Ministry of Health, Labour and Welfare, Food Additives
https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/shokuhin/syokuten/index_00012.html - Consumer Affairs Agency, Food Labelling
https://www.caa.go.jp/en/policy/food_labeling/ - Food Safety Commission, Food Additive Expert Committee,
https://www.fsc.go.jp/senmon/tenkabutu/ - Japan Food Chemical Research Foundation, Food Additives
https://www.ffcr.or.jp/en/index.html#news - Japan Flavor & Fragrance Materials Association
http://www.jffma-jp.org/english/